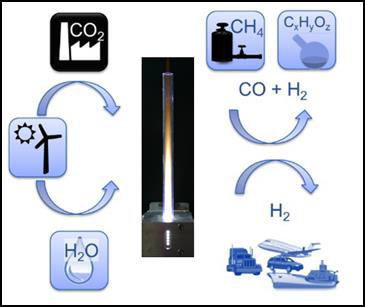
In periods with high output, electrical energy extraction from renewable energy sources (e.g. photovoltaics, wind and water) can easily exceed the load and gets wasted. For maintaining stability of the public mains supply, surplus energy from renewable sources has to be stored, which poses a big challenge. Power-to-X is a general term summarizing technologies for conversion of this kind of surplus energy from renewable sources into matter that either can be stored and reconverted when required, or that will serve as basic materials for the production of e.g. more complex substances in chemical industry or synthetic fuels replacing fossil fuels in the transport sector. Figure 1 shows some examples of Power-to-X applications based on microwave plasma technology.
Examples of Power-to-X applications based on microwave heating and microwave plasma technology are presented in the following chapters.
Microwave-driven depolymerization processes (pyrolysis) are ready to be used for Power-to-Liquid applications, e.g. production of bio-fuel. Standard applications can be found in biomass-to-liquid or waste-to-liquid plants.
Microwave-assisted pyrolysis processes are well suited to recycle a large variety of carbonaceous waste fractions such as tires, sewage sludge, agricultural waste, waste wood, electronic scrap, cables, plastic waste etc. to liquid fuels like heavy
oil, diesel, gasoline and jet fuel. The processes often rely on rapid heating of waste in an oxygen-free environment. The feedstock is introduced into the pyrolysis reactor of Figure 2 through air locks purged with inert gas to prevent oxygen to enter the reactor. Then it is heated by means of microwaves to a temperature level just beyond the threshold for separation of solid and volatile compounds of the feedstock. In a subsequent condensation process, part of the volatile compounds can be transformed into fluids for additional separation. At the end of the pyrolysis process, bio fuels, oils and monomers are extracted via condensers and separated from the remaining char.
Microwave heating is very homogeneous due to the high penetration depth of microwaves into the feedstock, providing a low temperature gradient from the surface to the core of the feedstock.

Storage of surplus electrical energy from renewable sources is a crucial factor for maintaining stability of the public mains supply. Carbon dioxide (CO2) conversion is a promising approach for storing surplus renewable energy. The concept of CO2 conversion is based on splitting CO2 into oxygen (O) and carbon monoxide (CO) radicals in an atmospheric pressure microwave plasma process, see Figure 3. Carbon monoxide (CO) is an industrial gas, which has numerous applications in chemical manufacturing. It can be converted into base chemicals and chemical energy stores such as methanol or methane in existing infrastructures using conventional chemical processes.
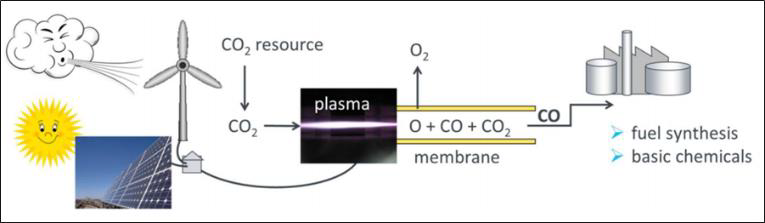
CO2 conversion can be efficiently performed with a high-power microwave plasma torch using excess electrical energy from regenerative sources. By separation of the oxygen from the gas mixture, for example via a perovskite membrane – as shown in Figure 4 –, the remaining CO gas can be utilized for the conversion into syngas or higher hydrocarbons. Hence, a zero emission carbon cycle can be established.
The process can be applied wherever CO2 is produced in enriched form: in combustion processes in power plants, in the cement and glass industries, and in breweries where CO2 is a by-product of alcoholic fermentation.
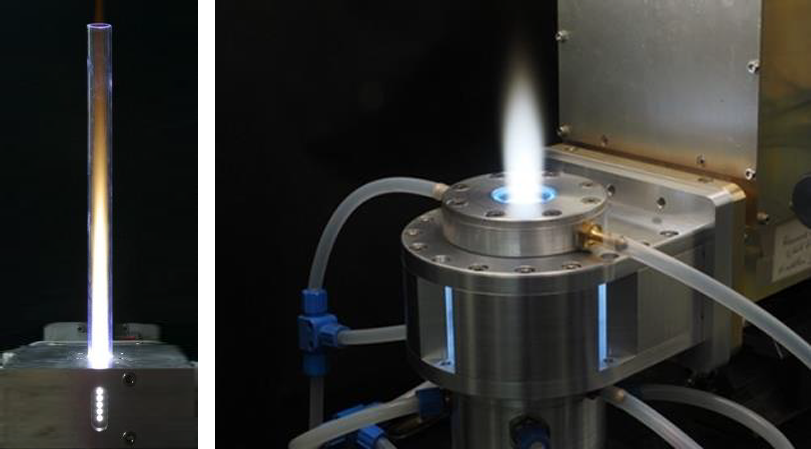
The new generation of MUEGGE’s microwave powerheads, generators and tuning elements enable compact plasma sources at atmospheric pressure for surface and volume treatment. The Atmospheric Plasma Source (APS) from MUEGGE operated at the microwave frequencies of 2.45 GHz and of 915 MHz, respectively, is a feasible tool for production of syngas via CH4 and CO2 conversion. Figure 5 shows microwave plasma torches operated with 6 kW (left) and 3 kW (right) of microwave power, respectively. Microwaves with a frequency of 2.45 GHz are fed into the plasma source resulting in a high field concentration in the middle of the cavity. In this region, the plasma is ignited and sustained. Several kilowatts of microwave power can be injected into the plasma, resulting in gas temperatures of up to 3500 K determined by optical emission spectroscopy.
MUEGGE’s microwave plasma torches are igniting at atmospheric pressure and generate a contact-free plasma while ensuring stable operation in a wide parameter range concerning type of gas, working gas flow and microwave power. Whatever microwave frequency is selected, 2.45 GHz or 915 MHz, microwave plasma torches from MUEGGE are well suited for both synthesis of special gases and supporting chemical reactions with highly reactive gas species, which is key for many Power-to-X applications, e.g. Power-to-Chemicals and Power-to-Gas.
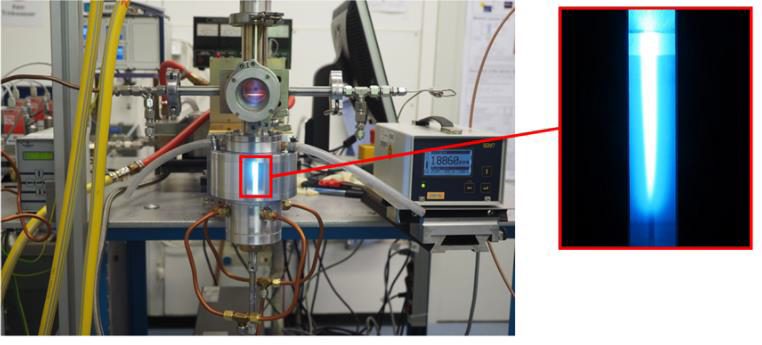
A high-power downstream-plasma-source operated at the microwave frequency of 915 MHz at a few mbar is presented in Figure 6. This device is characterized by its high microwave power input of up to 75 kW, enabling the treatment of high gas flows.
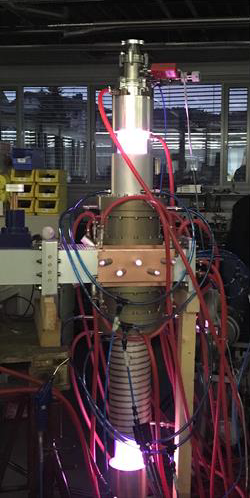
Efficient CO2 dissociation combined with high conversion rates of CH4 – being a prominent example of Power-to-Gas applications – can easily be performed by such highly energetic microwave plasma sources. The H2/CO mole ratio of the syngas is relatively easy to control by adjusting the ratio of CO2/CH4 in the feeding process. Furthermore, the syngas produced by this sources is not only usable for the production of e.g. acetic acid or methyl formate, but also satisfies the H2/CO mole ratio required for the production of various substances when combined with wet syngas processes. The process efficiency can be significantly enhanced by additional application of a suitable catalyst.
The same microwave plasma source-equipment can be used to create a plasma environment to decompose alcohols. When introduced into a water vapor plasma discharge, methanol and ethanol, respectively, decompose to hydrogen. In fact, nearly 100% decomposition of methanol can be achieved in an atmospheric microwave plasma process. The steam reforming reaction
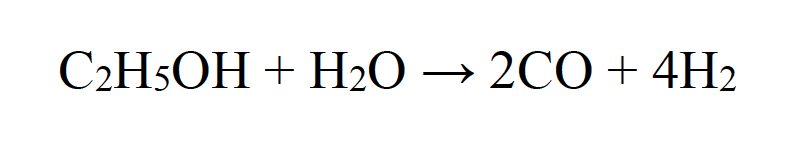
is the most likely source of H2 production in this case, which is confirmed by the fact that no formation of solid carbon was observed. This kind of atmospheric plasma process by application of a microwave plasma torch is very efficient for H2 production from methanol and ethanol, respectively.
A carbon-free, circular economy is required to decrease greenhouse gas emissions. Hydrogen economy is a commonly proposed alternative to the carbon-based economy. However, storing and transporting hydrogen is difficult. Ammonia (NH3) as a carbon-free hydrogen carrier is a relatively safe alternative to hydrogen. Especially in the long term, it is more economic to store ammonia than hydrogen. High-energy electrons and ions as well as highly reactive radicals in an atmospheric microwave torch plasma significantly enhance chemical kinetics. However, the high level of activation energy necessary for the dissociation of the nitrogen molecule is rate limiting in ammonia production. Plasma catalysis uses the synergy effects of plasmas and catalysts for the synthesis of various compounds. In case of ammonia synthesis, plasma catalysis helps to overcome the rate-limiting step of nitrogen dissociation prior to NHX formation. In this perspective, the combination of plasma and catalyst for using their synergies shows high benefits in ammonia production from renewable energy sources.
In general, Power-to-X combines all available options for the effective and flexible use of surplus energy from renewable sources. Power-to-X technologies based on microwave heating and microwave plasma processes are innovative solutions for conversion of electrical energy from renewable sources into material resources such as hydrogen, carbon monoxide, and synthetic gases for storage and recycling – e.g. conversion of electrical energy into gaseous or liquid fuels or chemicals for long-haul trucking, shipping and aviation. Therefore, Power-to-X contributes to the objective of decarbonising the energy systems, and at the same time helps to reduce the proportion of fossil fuels in the key leading markets of transport, travel and chemicals, thus generating ecological, economical and social benefits.

received his Chemistry diploma and PhD degree from the Ludwig-Maximilians-University, Munich, Germany. He has > 20 years of semiconductor experience, working on various positions in etch&strip, CVD and RTP. He joined the MUEGGE group in October 2016 and he currently heads Gerling Applied Engineering, the US branch of MUEGGE GmbH.
MUEGGE Group
Hochstrasse 4 – 6
64385 Reichelsheim
Germany
Gerling Applied Engineering, Inc.
P.O. Box 580816
Modesto, CA 95358-0816
USA
Sie benötigen eine besondere Lösung für Ihren individuellen Prozess?
Sie benötigen eine besondere Lösung für Ihren industriellen Prozess?
Sie sehen gerade einen Platzhalterinhalt von Vimeo. Um auf den eigentlichen Inhalt zuzugreifen, klicken Sie auf die Schaltfläche unten. Bitte beachten Sie, dass dabei Daten an Drittanbieter weitergegeben werden.
Mehr InformationenSie sehen gerade einen Platzhalterinhalt von YouTube. Um auf den eigentlichen Inhalt zuzugreifen, klicken Sie auf die Schaltfläche unten. Bitte beachten Sie, dass dabei Daten an Drittanbieter weitergegeben werden.
Mehr InformationenSie müssen den Inhalt von reCAPTCHA laden, um das Formular abzuschicken. Bitte beachten Sie, dass dabei Daten mit Drittanbietern ausgetauscht werden.
Mehr InformationenSie sehen gerade einen Platzhalterinhalt von Google Maps. Um auf den eigentlichen Inhalt zuzugreifen, klicken Sie auf die Schaltfläche unten. Bitte beachten Sie, dass dabei Daten an Drittanbieter weitergegeben werden.
Mehr Informationen